Rational design of small molecules
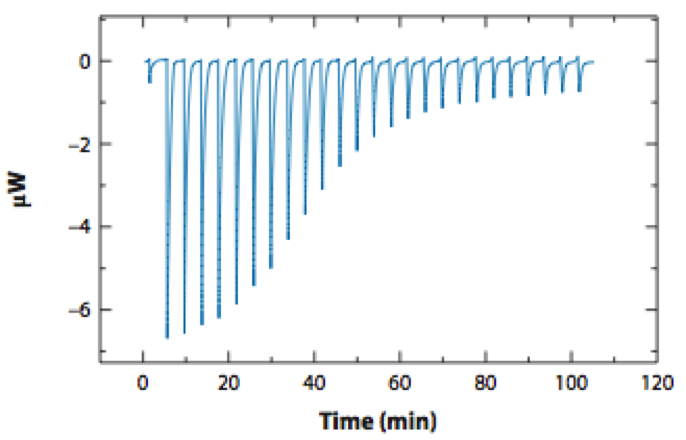
/To enable truly rational computational design of small molecules, we are developing new algorithms and open source tools for alchemical free energy calculations that provide a rigorous but practical approach to the quantitative prediction of small molecule binding affinities and other physicochemical properties of relevance to ADME-Tox (such as partition coefficients, serum binding, and off-target affinities).
Read More